I found this article on Oral Tren thought I'd put it up:
Note the extremely high Anabolic/Androgenic Ratio: approximately 12 000/6 000 (test E is 100/100).
Pharmaceutical Name: Methyltrienolone
Chemical Structure: 17beta-Hydroxy-17-methylestra-4,9,11-trien-3-one
Chemical Formula: C19H24O2
Molecular Weight: 284.3974
Active Life: four to six hours
Anabolic/Androgenic Ratio: approximately 12 000/6 000
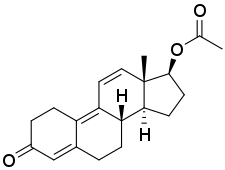
Methyltrienolone is essentially the same compound as trenbolone, but with the addition of 17 alpha alkylation, allowing it to remain active after oral administration.
In simple terms, methyltrienolone offers all the advantages of trenbolone in an orally active form. However, the increased benefit comes with a significant price in terms of hepatoxicity, as demonstrated below.
Similar to trenbolone, methyltrienolone has a remarkably strong binding affinity for the androgen receptor, even surpassing that of testosterone.
This supports the claim that trenbolone is highly anabolic. As binding to the androgen receptor activates the anabolic mechanisms responsible for muscle growth.
Androgen receptors are also present in adipose tissue, and when stimulated by compounds like
Tren Oral. They can promote higher-than-normal lipolytic action (1).
This means that the drug not only helps to build muscle, but also aids in fat burning.
Another distinctive characteristic of methyltrienolone is its anti-catabolic abilities. By binding with receptors that interact with glucocorticoid hormones, which are catabolic hormones (2), methyltrienolone can inhibit cortisol and other catabolic hormones in the body.
This makes it ideal for users aiming to reduce body fat. As the compound helps minimize muscle wasting when running a calorie deficit.
Oral Tren Use & Dosing
Oral Trenbolone was originally developed by a French pharmaceutical company, but never commercially produced due to its severe hepatoxic effects. This indicates the true harshness of the drug.
Therefore, users should exercise caution and limit their cycles to short periods. Excluding other highly hepatoxic steroids like 17 alpha alkylated oral steroids.
For cycle duration, most users limit themselves to four weeks or even shorter. Any extension of this time requires continuous blood tests by a doctor, regardless of the planned duration. Individual response and liver function should also be considered.
Regarding dosing, since little research has been conducted on Oral Tren, anecdotal information guides the dosage recommendations.
Normal doses of Oral Trenfor males range from 400-800 micrograms. Females are not advised to use this drug.
Higher doses have been reported, but this increases the risk of negative side effects, including temporary liver conditions like jaundice. Users should be cautious when starting or increasing the dosage.
Oral Tren Side Effects/Risks
Methyltrienolone, similar to trenbolone, does not exhibit estrogenic activity, thus alleviating concerns about estrogenic side effects. However, it is important to note that Oral Tren is highly androgenic and may result in side effects such as prostate enlargement, oily skin/acne, and potential hair loss.
Due to its androgenic nature, women are strongly advised against using this compound as it can lead to virilizing effects.
While methyltrienolone lacks estrogenic side effects. It functions as a progestin and can bind to progesterone receptors, potentially causing breast growth and lactation. Additionally, methyltrienolone can enhance estrogenic side effects caused by other aromatizing drugs. To mitigate these effects, users can consider using ancillary drugs like:
- bromocriptine,
- vitamin B6,
- cabergoline, or letrozole to lower prolactin and progesterone levels.
Trenbolone Oral, being a progestin, has a significant impact on natural testosterone production. Similar to trenbolone and nandrolone, methyltrienolone can suppress testosterone production for an extended period. To avoid sexual dysfunction, libido problems, or mental side effects associated with low testosterone. It is advisable for users to combine testosterone with Oral Trenbolone , even during short cycles.
While some animal studies suggest that methyltrienolone may increase libido in certain cases, others have shown the opposite effect. Thus, testosterone supplementation is recommended as a precautionary measure.
Notably, the company that originally developed Oral Tren deemed it too hepatotoxic for commercial human use.
Methyltrienolone is particularly harsh in terms of hepatoxicity, leading to elevated liver values with moderate use. Liver damage should be considered a significant risk if doses or duration of usage exceed accepted standards or if the user has existing liver abnormalities.
Therefore, concurrently using other 17 alpha alkylated steroids with methyltrienolone, or using them around the same time, is a risk that should be avoided.
Helpful Links
Trenbolone Enanthate vs Trenbolone Acetate
References
1. Sjogren J, Li M, Bjorntorp P. Androgen hormone binding to adipose tissue in rats. Biochim Biophys Acta. 1995 May 11;1244(1):117-20.
2. Ho-Kim MA, Tremblay RR, Dube JY. Binding of methyltrienolone to glucocorticoid receptors in rat muscle cytosol. Endocrinology. 1981
Nov;109(5):1418-23.
3. Lax ER, Baumann P, Schriefers H. Changes in the activities of microsomal enzymes involved in hepatic steroid metabolism in the rat after administration of androgenic, estrogenic, progestational, anabolic and catatoxic steroids. Biochem Pharmacol. 1984 Apr 15;33(8):1235-41.
4. Dube JY, Tremblay RR, Chapdelaine P. Binding of methyltrienolone to various androgen-dependent and androgen-responsive tissues in four animal species. Horm Res. 1976;7(6):333-40.
5. Sodersten P, Gustafsson JA. Activation of sexual behaviour in castrated rats with the synthetic androgen 17 beta-hydroxy-17 alpha-methyl-estra-4,9,11-triene-3-one (R 1881). J Endocrinol. 1980 Nov;87(2):279-83.
6. Baum MJ, Kingsbury PA, Erskine MS. Failure of the synthetic androgen 17 beta-hydroxy-17 alpha-methyl-estra-4,9,11-triene-3-one (methyltrienolone, R1881) to duplicate the activational effect of testosterone on mating in castrated male rats. J Endocrinol. 1987 Apr;113(1):15-20.